

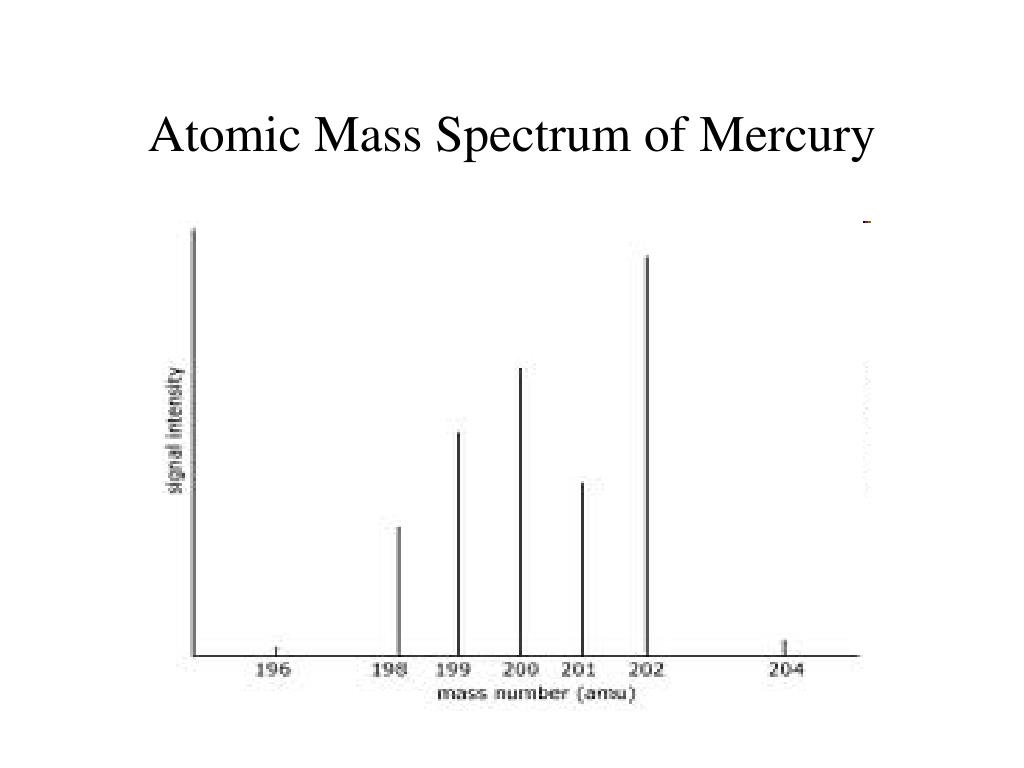
By definition, an atom of carbon with six neutrons, carbon-12, has an atomic mass of 12 amu. The atomic weight of cerium is approximately 30. You will need to refer to a periodic table for proton values. The atomic mass of a single atom is simply its total mass and is typically expressed in atomic mass units or amu. Answer to 11 The atomic weight of the element mercury, in atomic mass units amu, is approximately 200 amu. In this notation, the atomic number is not included. Symbol-mass format for the above atom would be written as Cr-52. The average atomic mass (sometimes called atomic weight) of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen.
The "A" value is written as a superscript while the "Z" value is written as a subscript.
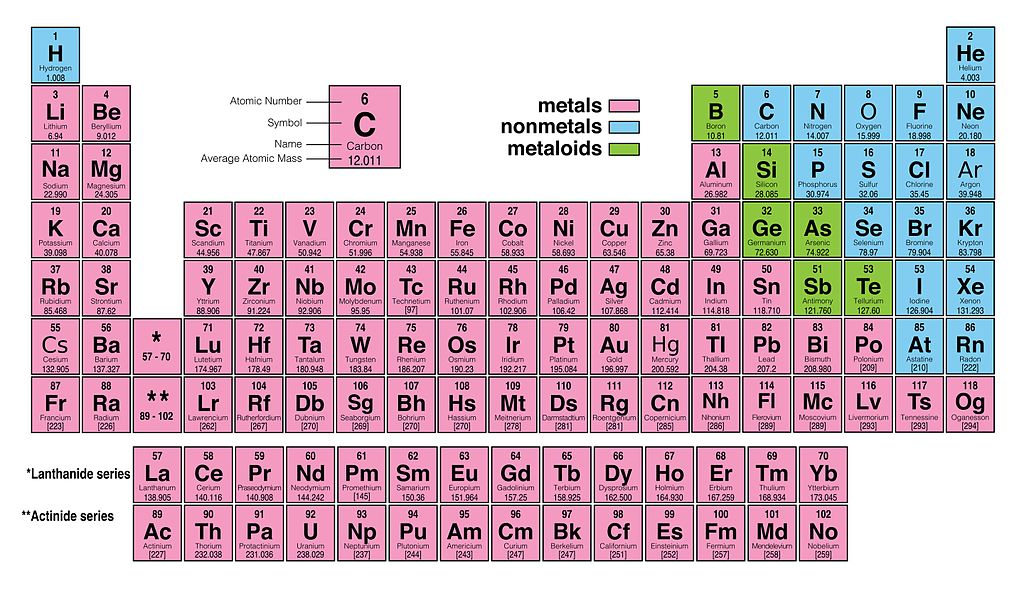
Both the atomic number and mass are written to the left of the chemical symbol. It is also known as quicksilver and was formerly named hydrargyrum ( / hadrrdrm / hy-DRAR-jr-m) from the Greek words hydro (water) and argyros (silver). Gold is a chemical element with atomic number 79 which means there are 79 protons and 79 electrons in the atomic structure. The composition of any atom can be illustrated with a shorthand notation called A/Z format. Mercury is a chemical element with the symbol Hg and atomic number 80.


 0 kommentar(er)
0 kommentar(er)
